By Daniel Dunaief
Monday, June 23, marked the beginning of a new and exciting frontier. Using the largest digital camera ever built for astronomy, the Vera C. Rubin Observatory shared its first images after a journey from conception to reality that lasted over two decades.
Located in the Cerro Pachón mountaintop in Chile because the area is dry, high and dark, the telescope and camera started its 10-year mission to share images of the sky.
Viewers at over 350 watch parties in the United States and around the world awaited these pictures, including with gatherings at Stony Brook University and Brookhaven National Laboratory.
The state-of-the-art camera did not disappoint.
The Rubin Observatory, which can take images with a field of view of the sky that are the equivalent of 40 moons, discovered 2,400 asteroids that no one has ever seen before. And that’s just the tip of the iceberg. By the time the Observatory has collected all the data the public can view, the camera is expected to find over five million asteroids.
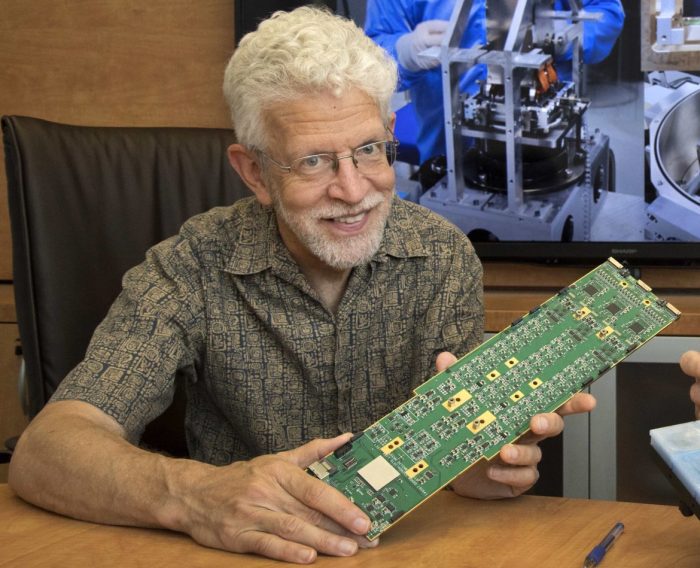
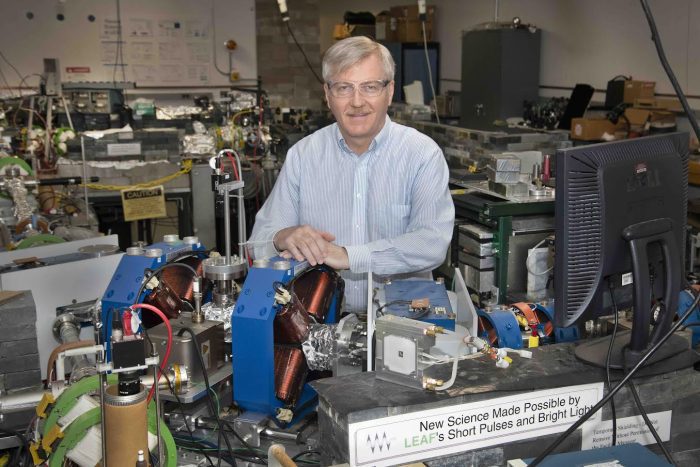
“Most of the asteroids are too faint to have been found” with previous technology, said Paul O’Connor, senior physicist at Brookhaven National Laboratory who has been working on the camera since 2002.
Simon Birrer, Assistant Professor in the Department of Physics and Astronomy at Stony Brook University, attended a watch event at the university with some 50 to 60 other excited members of the college community.
“Knowing that the instrument is capable and what it was promised to do and seeing it all coming together, sharing the excitement with so many other people is very exciting,” said Birrer.
By looking at the night sky over the course of just a few days, the observatory was able to offer a time lapse view of the movement of these asteroids.
“You can look and see the trail of thousands of things that are completely new,” said Birrer.
Indeed, in addition to seeing asteroids and other objects both near and far, the Rubin Observatory can study dark matter and dark energy, map the Milky Way, and observe transient events.
“We’re entering a golden age of American science,” Harriet Kung, acting director of the DOE’s Office of Science, said in a statement. “NSF-DOE Rubin Observatory reflects what’s possible when the federal government backs world-class engineers and scientists with the tools to lead.”
The first images generated considerable excitement in the scientific community and on campuses around the world.
“It’s a new frontier for sure,” said O’Connor. “We’ve been working on this project for all these years. It was easy to get students interested.”
Anja von der Linden, Associate Professor in Physics and Astronomy at Stony Brook and a member of the LSST Dark Energy Science Collaboration since its inception in 2012, viewed the images from Germany, where she is visiting her parents on vacation with her young daughter.
She works on clusters of galaxies and was delighted to see the Virgo cluster online.
“The image is so large and [viewers] can also see much more distant galaxies,” said von der Linden. Viewers are able to scroll around and zoom in and out to see details in these “beautiful images.”
Von der Linden echoed the sentiment from one of the officials who shared the first images, suggesting that the data and information from the observatory are available for astronomers and scientists, but also for the public, helping them explore the night sky.
“It’s quite remarkable,” she said. “I look forward to seeing how the public engages.”
The Rubin Observatory will see “everything that changes, explodes, and moves,” said von der Linden.
A little bit of pride
In addition to scientists like O’Connor and Anže Slosar, group leader of the Cosmology & Astrophysics Group, BNL recruited close to two dozen interns to help with the work.
“There’s a lot of inherent curiosity about the cosmos,” O’Connor said. “When people hear that they could participate in doing research that could lead to lead to a better understanding of it, we had to turn interns away.”
O’Connor worked with the charge-coupled device modules, which are the digital film of the camera. The Rubin Observatory, with its 3.2 gigapixel focal plane, relies on 189 custom-designed CCD sensors to achieve its resolution.
“I feel a little bit of pride,” said O’Connor, who didn’t expect to be working on astronomical instruments when he came to BNL. “I was a tiny, little part of a giant team that’s worked so long. When you see the final project, it’s a good feeling.”
Seeing the invisible
At the same time that the Rubin Observatory can find asteroids that had previously gone undetected, it can also help detect dark energy and dark matter.
Only five percent of the universe comes from visible matter, with about 70 percent coming from dark energy and 25 percent coming from dark matter.
Dark energy describes why the universe continues to expand after the Big Bang, rather than slowing down, the way a ball thrown into the air does before it falls, von der Linden explained. Researchers study dark matter, meanwhile, by observing the way light from distant galaxies bends when it travels towards Earth, as the gravitational force of the matter affects it on its path.
Von der Linden said she has already started using some of the commissioning data to test Rubin’s capabilities to do weak gravitational lensing. Weak gravitational lensing involves slight shifts in images caused by the gravitational influence of other matter that require many galaxies to detect.
“The work we’re doing now is very much a test case, which we will then take and apply to a much larger data set,” she said.
Inspiring future scientists
The images and the data, which the US, the UK and France will process, has the potential not only to answer scientific questions, but also to encourage and inspire future researchers.
The Rubin Observatory has a “very comprehensive education and public outreach component,” von der Linden said. “From the beginning, it has been built with the intention that the public is suppose to interact with the data and be part of the scientific story.”
If teachers use this in the classroom to show students the beautiful and intriguing night sky, “I would think this will lead some students to consider pursuing” careers in these sciences. “I hope that we’re going to get more junior scientists who will be part of Rubin.”
To see images from the observatory, visit https://rubinobservatory.org.