By Daniel Dunaief

Researchers have long connected exposure to violence, particularly at a younger age, to expressions of violence as people age.
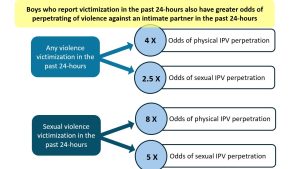
In a recent study of boys between the ages of 15 and 19 years old published in the journal PLOS One, lead author Rachel Kidman, Core Faculty in the Program in Public Health and Associate Professor in the Department of Family, Population and Preventive Medicine in the Renaissance School of Medicine at Stony Brook University, however, has made a connection between various types of violence adolescent boys witnessed or were subjected to and violence within 24 hours towards intimate partners.
“Those boys who experience violence that day are much more likely to act out and engage in intimate partner violence against their girlfriend or boyfriend,” said Kidman.
Adolescent boys are getting “trapped in a cycle” in which they experience and then perpetuate violence, Kidman said.
In parts of Africa, in particular, intimate partner violence could be particularly dangerous as the rate of HIV infection — the virus that causes AIDS — is higher.
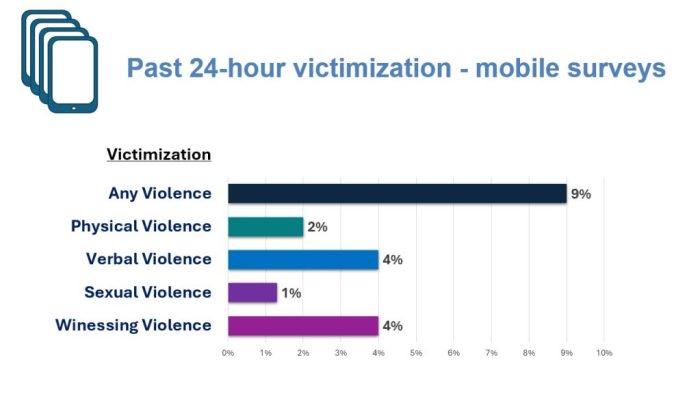
This study, which was conducted with 498 adolescents living in Soweto, South Africa who responded to cell phone surveys from November of 2020 to June of 2022, expands the understanding of the development of abuse and violence.
Amy Hammock, Associate Professor in the School of Social Welfare at Stony Brook University, who has collaborated on research with Kidman but was not a part of this paper, lauded the work for its “strong design” which relies on surveys that measure violence within 24 hours. The surveys allow for “more accuracy in reporting” than a typical question about violence within the last year.
“Many of the boys in the sample experienced significant violence, both at the community level and the interpersonal level,” Hammock explained.
Previous evidence indicates that men who experienced or witnessed childhood trauma or domestic violence between their parents perpetrate intimate partner violence at higher rates.
“We don’t have a lot of evidence of what happens during the teenage years,” said Kidman. “This could be setting the course for relationships in the future.”
Adolescents could be responding to triggers and may not know how to cope with their own emotions, which leads to their own violent actions.
Working with boys
Public health programs typically focus on ways to protect people in relationships, often women, against violence, by encouraging them to take self-defense classes and to recognize the signs of an abusive relationship. Many of these approaches place the onus on the victim, which seems too narrow, Kidman said. As a next step, “we can work with boys, acknowledge the trauma.”
By exploring the link between physical, verbal and sexual violence perpetrated against adolescent boys, researchers are taking a first step towards developing methods that might help boys cope with their own emotions without lashing out at their partners.
“We need to learn more to design the right intervention,” Kidman explained.
Breaking the cycle
Kidman chose to work with adolescents in this area of South Africa in part because she had forged connections with researchers in the area in previous studies and in part because of the high levels of HIV and violence for an underserved population.
She feels it’s important to understand the epidemics of violence in low and middle income countries.
“The area we work in has a history of apartheid and racial and economic segregation and a long history of violence and a high rate of HIV,” Kidman said.
To be sure, ideally, these adolescents wouldn’t experience any violence. Many of the adolescents who participated in this study experienced intimate partner violence directed against them as well, which could be initiated by a girlfriend or be used by a girlfriend in self defense.
“Some of this may be in the context of bi-directional violence in the relationship,” said Kidman.
Participants in this study could ask to speak with a counselor. Kidman appreciates the adolescents who shared personal and painful details their lives.
“These are not easy topics to talk about and they get a lot of credit for being so open,” she said. “This gives us insights into how we can help.”
Meaningful semester abroad
The direction Kidman’s research has taken springs from research she did during her undergraduate training at Swarthmore College, particularly during a semester abroad in Zimbabwe. She was interested in the social dimensions of HIV. During her master’s degree at the Harvard School of Public Health, she studied the survival and education of orphaned children.
As she conducted that research, Kidman considered the many adversities affecting children, including violence, child abuse, neglect, and living with someone who has substance abuse problems, among others. The current project is exploring how these experiences during childhood and adolescence, including child abuse, verbal violence and bullying, affect youth and their behavior towards intimate partners.
Indeed, when youth with HIV experience violence, they sometimes don’t take their medications, which increases the health risks to themselves and their partners.
Role models
Born and raised in Portland, Maine, Kidman received considerable support for her broader interests in the world from her parents Joan and Bruce Kidman.
“When I announced that I was going to Zimbabwe for the semester, they were absolutely on board,” said Kidman.
Indeed, both of her parents, who met in college, worked together for the Peace Corps in Micronesia after they graduated. When she was in college, Kidman was eager to learn about a different culture.
Kidman and her husband Sean Clouston, Professor in the Department of Family, Population and Preventive Medicine in the Renaissance School of Medicine at SBU, live in Stony Brook with their 10 and 12-year old children Riley and Quinn.
As for her work, Kidman suggested numerous questions remain. She urged further studies that could assist with preventing violence and supporting those people who can be victims and perpetrators.