APDA Long Island Information and Referral Centers supports area Parkinson’s community in April and year-round.
April is Parkinson’s Disease Awareness Month, and the American Parkinson Disease Association (APDA) will commemorate the month with a “Did You Know?” campaign that will educate the public about Parkinson’s disease (PD) – highlighting everything from statistics and symptoms to personal stories and ways to get involved – while also helping those affected by PD feel empowered with the resources and support they need.
Through a nationwide network of Chapters and Information & Referral (I&R) Centers, APDA works every day to provide the support, education, and research that will help everyone impacted by PD live life to the fullest. The APDA I&R Centers at St Charles Hospital and Catholic Health Ambulatory Center Commack supports people living with PD throughout Long Island NY and beyond, as well as their care partners and loved ones by helping them assemble the resources, support, and medical expertise they need to feel more empowered, connected, and optimistic.
Throughout Parkinson’s Disease Awareness Month (and always), the APDA I&R Centers at St Charles Hospital and Catholic Health Ambulatory Center Commack have a variety of programs and events planned, with many ways for people to get involved. April’s activities include an in person PD Educational Lecture at St Charles Hospital, a Lunch and Learn, Support Groups for both PWP and their Care partners and family members and much more. Additionally, throughout the month, APDA will share educational information and resources on all APDA social media channels using #DidYouKnow.
With approximately one million people living with PD in the United States –65,000 of which are in New York State – and 90,000 new diagnoses every year, it is critical to engage, inform, and support the PD community and raise public awareness about the disease. Parkinson’s Disease Awareness Month is the perfect time to shine a spotlight on this issue.
“With a new diagnosis every six minutes, nearly 7,200 people in this country will learn they have PD in April alone,” said Julie Garofalo, RN, APDA I& R Centers Coordinator at St. Charles Hospital and Catholic Health Ambulatory Center in Commack. “Here on Long Island, we are the boots on the ground. From support groups and exercise classes to educational events and access to PD experts, the APDA Long Island I&R Centers are here for every member of our local PD community, working tirelessly to help make their journey more positive.”
Beyond Long Island, APDA offers extensive virtual programming and a robust resource library – with many resources available in Spanish and Mandarin/Simplified Chinese – to ensure that all members of the PD community have access to high-quality information and services no matter where they live and to help them to feel connected to the community even from a distance. From popular webinar series like Dr. Gilbert Hosts, Unlocking Strength Within, and Let’s Keep Moving with APDA to a variety of virtual exercise and movement classes, there is something for everyone.
Support from the public is crucial, and Parkinson’s Disease Awareness Month is an especially meaningful time to take action to help those coping with this progressive neurodegenerative movement disorder. People can support by raising awareness of PD and/or by making a donation @ www.apdaparkinson.org that will enable APDA to continue their critical work and fund research that will lead to better treatments and ultimately, a cure. Every effort makes a difference.
The APDA Long Island I&R Centers at St Charles Hospital and Catholic Health Ambulatory Center Commack offers a wide range of Parkinson’s disease programs, resources, education, and support. To learn more, visit www.apdaparkinson.org/ny or [email protected] or call 631-862-3560
About the American Parkinson Disease Association:
The American Parkinson Disease Association (APDA) is a nationwide grassroots network dedicated to fighting Parkinson’s disease (PD) and works tirelessly to assist the more than one million people with PD in the United States live life to the fullest in the face of this chronic, neurological disorder. Founded in 1961, APDA has raised and invested more than $282 million to provide outstanding patient services and educational programs, elevate public awareness about the disease, and support research designed to unlock the mysteries of PD and end this disease. To join in the fight against Parkinson’s disease and to learn more about the support APDA provides nationally through a network of Chapters and Information & Referral (I&R) Centers, as well as a national Research Program and Centers for Advanced Research, please visit us at www.apdaparkinson.org.












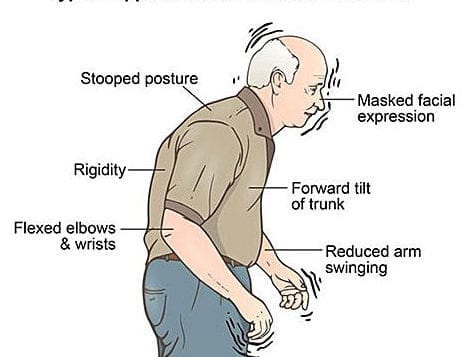

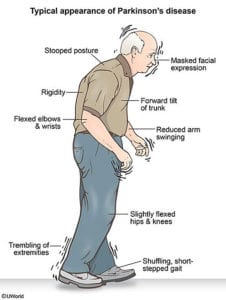 When we typically think of using CoQ10, a coenzyme found in over-the-counter supplements, it is to compensate for depletion from statin drugs or due to heart failure. Doses range from 100 to 300 mg. However, there is evidence that CoQ10 may be beneficial in Parkinson’s at much higher doses.
When we typically think of using CoQ10, a coenzyme found in over-the-counter supplements, it is to compensate for depletion from statin drugs or due to heart failure. Doses range from 100 to 300 mg. However, there is evidence that CoQ10 may be beneficial in Parkinson’s at much higher doses.


