Prestigious honor recognizes development of widely used protein- and RNA-production platform
F. William Studier, a senior biophysicist emeritus at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory, has won the 2024 Richard N. Merkin Prize in Biomedical Technology [https://merkinprize.org/] for his development in the 1980s of an efficient, scalable method of producing RNA and proteins in the laboratory. His “T7 expression” technology can be used to make large quantities of nearly any RNA or protein and has been for decades, and continues to be, a mainstay of biomedical research and pharmaceutical production. Studier’s approach has been used to produce numerous therapeutics, diagnostics, and vaccines — including the COVID-19 mRNA vaccines credited with extending millions of lives in recent years [see: https://www.bnl.gov/newsroom/
“F. William Studier’s brilliant work on the T7 system transformed biomedicine, saving millions of lives globally and improving the chances for further research that will change healthcare delivery,” said Dr. Richard Merkin, CEO and founder of Heritage Provider Network, one of the country’s largest physician-owned integrated health care systems. “His work exemplifies why I created this prize initiative that honors and showcases amazing innovators like Bill. I’m honored to be celebrating his remarkable achievements.”
The Merkin Prize, inaugurated in 2023, recognizes novel technologies that have improved human health. It carries a $400,000 cash award and is administered by the Broad Institute of MIT and Harvard, one of the world’s leading biomedical research institutes. All nominations for the 2024 Merkin Prize were evaluated by a selection committee composed of nine scientific leaders from academia and industry in the U.S. and Europe. Studier will be honored in a prize ceremony held on Sept. 17, 2024.
“The T7 system has been influential in biomedicine and has had important clinical implications for many years, but Bill Studier’s contribution to the field has really not been as celebrated as it ought to be,” said Harold Varmus, chair of the Merkin Prize selection committee. Varmus is also the Lewis Thomas University Professor at Weill Cornell Medicine, a senior associate at the New York Genome Center, and a recipient of the Nobel Prize in Physiology or Medicine for his work on the origins of cancer.
“Bill Studier’s development of T7 phage RNA polymerase for use in preparing RNA templates for multiple uses in research labs worldwide has been a truly revolutionary technical advance for the entire field of molecular biology,” said Joan Steitz, the Sterling Professor of Molecular Biophysics and Biochemistry at Yale University.
“Today, virtually every protein you want to produce in bacteria is made with a T7 system,” said Venki Ramakrishnan of the Medical Research Council Laboratory of Molecular Biology in Cambridge, England, and a winner of the 2009 Nobel Prize in Chemistry. “There’s not a single molecular biology or biochemistry lab I know that doesn’t use T7.”
“This award is a great honor for Bill Studier, recognizing the significance of the research and technology he pioneered. It reinforces how basic research — asking fundamental questions about the way the world and everything in it works — can result in important and unexpected advances that continue to have impact even decades after the initial discoveries,” said Brookhaven National Laboratory Director JoAnne Hewett. “It is fabulous to see Bill recognized for his lifetime of work and the critical role it has played in biotechnology and medicine.”

Driven by basic biology
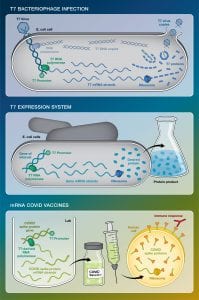
Studier grew up in Iowa and became fascinated with biophysics while an undergraduate at Yale University. Then, during graduate school at the California Institute of Technology in the early 1960s, he was introduced to bacteriophage T7, a virus that infects Escherichia coli bacteria. He wondered how T7 could so effectively and quickly take over E. coli, rapidly turning the bacterial cells into factories to produce more copies of itself. That question launched a career focused on the basic biology of T7.
“I’ve always been interested in solving problems,” Studier told Brookhaven National Laboratory in a 2011 profile [https://www.bnl.gov/newsroom/
When he joined Brookhaven Lab in 1964, Studier focused on sequencing the genes of the T7 bacteriophage and understanding the function of each of its corresponding proteins during infection of E. coli. By 1984, he and Brookhaven colleague John Dunn successfully identified and cloned the protein within T7 that was responsible for rapidly copying T7 DNA into many corresponding strands of RNA [see: https://www.pnas.org/doi/10.
Studier realized that the protein, called the T7 RNA polymerase, might be able to quickly and efficiently produce RNA from not only T7 DNA but also from the genes of any organism. If a gene was tagged with a special DNA sequence, known as the T7 promoter, then the T7 RNA polymerase would latch on and begin copying it. In 1986, Studier described this system in the Journal of Molecular Biology [https://pubmed.ncbi.nlm.nih.
“His work really illustrates that sometimes a remarkable technology can emerge not only from people trying to build technologies but from someone who is trying to use basic science to understand a fascinating biological phenomenon,” Varmus said.
Speeding science
Before Studier’s development of the T7 system, scientists who wanted to produce RNA or proteins generally inserted the genes into the natural E. coli genome and let the E. coli polymerase produce the corresponding RNA at the same time as the bacteria produced its own RNA and proteins. But the E. coli machinery was relatively slow, and scientists often ran into problems with the bacteria turning off their DNA-reading programs. T7 polymerase overcame both these problems: It was far faster, and E. coli had no built-in way to shut it off.
Within a few years, biologists had rapidly switched from their older methods to the T7 system for producing both RNA and proteins. When proteins are the desired end result, the E. coli molecular machinery for translating mRNA into proteins is used after the T7 system makes the RNA.
Studier continued studying the T7 polymerase and promoter, fine-tuning the system for years and publishing new improved versions as recently as 2018.
As of 2020, the T7 technology had been cited in more than 220,000 published studies, with 12,000 new studies using the technology published each year. There are more than 100 different versions of the T7 technology available commercially and 12 patents in Studier’s name related to the system.
Making medicine
The T7 technology has also had immediate impacts in industry, with more than 900 biotech and pharmaceutical companies licensing it to produce therapeutics and vaccines.
In 2020, scientists used the T7 platform to produce enough mRNA for COVID-19 vaccines to vaccinate millions of people in the U.S. and around the world. With the T7 promoter placed next to the gene for the COVID-19 spike protein, the T7 polymerase could generate many kilograms of mRNA — the active molecule in the vaccines — at a time.
“I think it’s an incredible testament to this technology that, decades after its development, it’s still the go-to method for RNA and protein production,” said John Shanklin, a distinguished biochemist and chair of the Biology Department at Brookhaven National Laboratory, who considered Studier a mentor for many years.
Those who know Studier say the Merkin Prize is well-deserved; Studier changed the course of biomedicine while working quietly on basic science questions that interested him.
“Almost no one has heard of Bill Studier because he is a quiet, modest guy who had a small lab,” said Ramakrishnan, who worked with Studier at Brookhaven in the 1980s. “But he is an absolutely fantastic role model of what a scientist should be like.”
“He has flown under the radar and hasn’t been recognized for his accomplishments very much,” Shanklin agreed. “This is a well-deserved honor.”
Studier was also committed to guaranteeing access to his technology. When Brookhaven was in the process of licensing and commercializing the T7 system shortly after its development, Studier ensured that it remained free for academic labs while charging commercial licensing fees to companies.
F. William Studier earned a bachelor’s degree in biophysics from Yale in 1958, followed by a Ph.D. from the California Institute of Technology in 1963. He worked as a postdoctoral fellow in the Department of Biochemistry at Stanford University School of Medicine, and then he joined Brookhaven Lab’s Biology Department in 1964 as an assistant biophysicist. Over the years, Studier rose through the department’s ranks, receiving tenure in 1971 and becoming a tenured senior biophysicist in 1974.
He served as chair of the Biology Department from 1990 to 1999 and then returned to research. He also served as an adjunct professor of biochemistry at Stony Brook University. His achievements have been recognized by election to the American Academy of Arts and Sciences in 1990, the National Academy of Sciences in 1992, and as a Fellow of the American Association for the Advancement of Science in 2007. He retired from the Lab in 2015 and was named senior scientist emeritus. In 2018, he was elected as a Fellow of the National Academy of Inventors. He holds 15 patents of which nine have been licensed and commercialized, including those on the T7 system.
Studier’s research at Brookhaven Lab was supported by the DOE Office of Science.