The lingering effects of Lyme can be debilitating
By David Dunaief, M.D.

After a spring where we’ve spent more than our fair share of time indoors, summer’s heat is finally here. Many of us are taking advantage of the weather to enjoy day hikes and picnics along the shoreline or bike rides through wooded areas.
The summer’s heat also means that tick season is in full swing. This means we need to be aware of Borrelia burgdorferi, better known as the bacterium that causes Lyme disease. This bacterium is typically found in the deer tick, also known as the blacklegged tick.
What do deer ticks look like? They are small and can be as tiny as a pencil tip or the size of a period at the end of a sentence. The CDC.gov site is a great resource for tick images and other information related to Lyme disease.
If you have been bitten by a tick, you should remove it with forceps, tweezers or protected fingers (paper) as close to the skin as possible and pull slow and steady straight up. Do not crush or squeeze the tick; doing so may spread infectious disease (1). In a study, petroleum jelly, fingernail polish, a hot kitchen match and 70 percent isopropyl alcohol all failed to properly remove a tick. The National Institutes of Health recommend not removing a tick with oil (2).
When a tick is removed within 36 to 48 hours, the risk of infection is quite low (3). However, a patient can be given a prophylactic dose of the antibiotic doxycycline, one dose of 200 mg, if a bulls-eye rash — a red outer ring and red spot in the center — has not occurred, and it is within 72 hours of tick removal (4). Those who took doxycycline had significantly lower risk of developing the bulls-eye rash and thus Lyme disease; however, treatment with doxycycline did sometimes cause nausea.
Lyme Symptoms
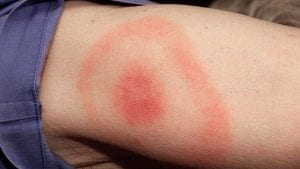
There are three stages of Lyme disease: early stage, where the bacteria are localized; early disseminated disease, where the bacteria have spread throughout the body; and late stage disseminated disease. Symptoms for early localized stage and early disseminated disease include the bulls-eye rash, which occurs in about 80 percent of patients, with or without systemic symptoms of fatigue (54 percent), muscle pain and joint pain (44 percent), headache (42 percent), neck stiffness (35 percent), swollen glands (23 percent) and fever (16 percent) (5).
Early disseminated disease may cause neurological symptoms such as meningitis, cranial neuropathy (Bell’s palsy) and motor or sensory radiculoneuropathy (nerve roots of spinal cord). Late disseminated disease can cause Lyme arthritis (inflammation in the joints), heart problems, facial paralysis, impaired memory, numbness, pain and decreased concentration (2).
Lyme carditis is a rare complication affecting 1.1 percent of those with disseminated disease, but it can result in sudden cardiac death (6). If there are symptoms of chest pain, palpitations, light-headedness, shortness of breath or fainting, clinicians should suspect Lyme carditis.
Preventing Lyme
According to the Centers for Disease Control and Prevention, we should wear protective clothing, spray ourselves with insect repellent that includes at least 20 percent DEET and treat our yards (4). Always check your skin and hair for ticks after walking through a woody or tall grassy area. Many of us on Long Island have ticks in the yard, so remember to check your pets; even if treated, they can carry ticks into the house.
Diagnosing Lyme
Lyme disease often can be diagnosed within the clinical setting or with a blood test. When it comes to serologic or blood tests, the CDC recommends an ELISA test followed by a confirmatory Western blot test (3). However, testing immediately after being bitten by a tick is not useful, since the test will tend to be negative, regardless of infection or not (4). It takes about one to two weeks for IgM antibodies to appear and two to six weeks for IgG antibodies (5). These antibodies sometimes remain elevated even after successful treatment with antibiotics.
Does chronic Lyme disease exist?
There has been a debate about whether there is something called “chronic Lyme” disease. The research, unfortunately, has not shown consistent results that indicate that it exists. In one analysis, the authors note that the definition of chronic Lyme disease is obfuscated and that extended durations of antibiotics do not prevent or alleviate post-Lyme syndromes, according to several prospective trials (7).
The authors do recognize that there are prolonged neurologic symptoms in a subset population that may be debilitating even after the treatment of Lyme disease. These authors also suggest that there may be post-Lyme disease syndromes with joint pain, muscle pain, neck and back pain, fatigue and cognitive impairment.
Ultimately, the IDSA (Infectious Diseases Society of America) argues in favor of recognizing post-Lyme disease syndromes, while the ILADS (International Lyme and Associated Diseases Society) believes chronic Lyme exists.
Regardless, the lingering effects of Lyme can be debilitating. This may be as a result of systemic inflammation (8). Systemic inflammation and its symptoms can be improved significantly with dietary and other lifestyle modifications.
The CDC recommends that physicians look beyond Lyme for other possible diagnoses before diagnosing someone with chronic Lyme disease (9).
Prevention is key to helping stem Lyme disease. If this is not possible, treating prophylactically when pulling off a tick is an important step. Contact your physician as soon as you notice a tick. If you have a bulls-eye rash and it is early, then treatment of antibiotics for two to three weeks needs to be started right away. If it is prolonged and disseminated, then treatment should be for approximately three to four weeks with antibiotics. If it has affected the central nervous system, then IV antibiotics could be needed.
References:
(1) Pediatrics. 1985;75(6):997. (2) nlm.nih.gov. (3) cdc.gov. (4) Clin Infect Dis. 2008;47(2):188. (5) uptodate.com. (6) MMWR. 2014;63(43):982-983. (7) Expert Rev Anti Infect Ther. 2011;9(7):787-797. (8) J Infect Dis. 2009;199(9:1379-1388). (9) JAMA Intern Med. online Nov. 3, 2014.
Dr. David Dunaief is a speaker, author and local lifestyle medicine physician focusing on the integration of medicine, nutrition, fitness and stress management. For further information, visit www.medicalcompassmd.com.














