By Daniel Dunaief
Do the birds on the Galapagos Islands, with their unique coloration, differently shaped beaks and specific nesting places, have anything to do with the cancer cells that alter the course of human lives?
For Mehdi Damaghi, Assistant Professor in the Department of Pathology at the Renaissance School of Medicine at Stony Brook University, the answer is a resounding, “Yes.”
Damaghi uses the same principles of evolutionary biology to understand how cancer, which resides within human genes, works to adapt, as it tries to win the battle to survive.
“What we try to understand is the Darwinian principals of cancer,” said Damaghi. Cancer “adapts and reprograms themselves” to their environment to survive.
Damaghi, who arrived at Stony Brook four months ago from Moffitt Cancer Center, plans to address numerous questions related to cancer. He recently received a $4 million grant from the Physical Science in Oncology program (PSON) through the National Institutes of Health/ National Cancer Institute. Working with cancer biologists, clinicians, and computational scientists, he plans to define and understand cancer’s fitness.
“We are trying to study the core evolution of cancer cells and the normal stroma around them,” said Damaghi. “We are looking at the evolution of the tumor and some of the host cells.”
Cancer biologists are trying to build mathematical and theoretical models to explore the playbook cancer uses when confronted with threats, either in the form of a body’s natural defenses against it or from therapies against which it can, and often does, develop resistance.
Treating cancer could involve using adaptive therapy, which could enable people to control and live with cancer longer, Damaghi suggested.
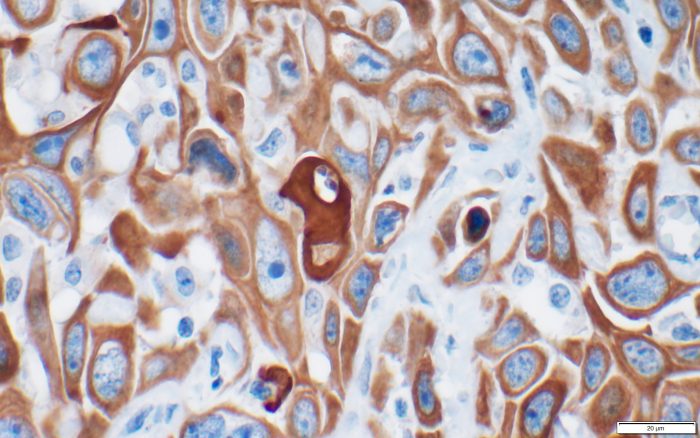
In studying cancer’s phenotype, or the way the disease is expressed and survives, he hopes to understand factors in the microenvironment. Many cancers, he reasons, become more problematic as people age. Indeed, centuries ago, cancer wasn’t as prevalent as it is today in part because life expectancy was shorter.
Damaghi also has an evolutionary model to explore metastasis, in which cancer spreads from one organ or system to other parts of the body. He is looking at the earliest stages of breast cancer, to see what factors some of these cancers need or take from the environment that enables them not only to develop into breast cancer, but also to spread to other systems.
Through the microenvironment, he is looking for biomarkers that might signal a potential tumor development and metastasis long before a person shows signs of an aggressive form of the disease.
“We look at the tumor as a part of a whole ecosystem that can have different niches and habitats,” he said. “Some can be hypoxic and oxidative, and others can be like a desert on Earth, where not much grows and then cancer evolves.”
Damaghi challenges cells in a culture or organoids, which are miniature, three-dimensional live models of human cells, with different microenvironmental conditions to see how they respond. He exposes them to hormones, immune cells, and hypoxic conditions.
“We try to understand what is the adaptation mechanism of cancer to this new microenvironment and how can we push them back to the normal phenotype,” he said.
Like other scientists, Damaghi has demonstrated that many of these cancer cells use sugar. Removing sugar caused some of the cancer to die.
Increasing the survival for patients could involve knowing what kinds of micro-environments cancer uses and in what order. Deprived of sugars, some cancers might turn to amino acids, dairy or other sources of food and energy.
Damaghi thinks researchers and, eventually, doctors, will have to approach cancer as a system, which might have a patient-specific fingerprint that can indicate the resources the disease is using and the progression through its various diseased stages.
Choosing Stony Brook
Damaghi appreciates the depth of talent in cancer sciences at Stony Brook University. He cited the work of Laufer Center Director Ken Dill and Cancer Center Director Yusuf Hannun. He also suggested that the Pathology Department, headed by Ken Shroyer, was “very strong.”
For their part, leaders at Stony Brook were pleased to welcome, and collaborate with, Damaghi. Hannun suggested Stony Brook recruited Damaghi because his research “bridges what we do in breast cancer and informatics.”
Shroyer, meanwhile, has already started collaborating with Damaghi and wrote that his new colleague’s focus on breast cancer “overlaps with my focus on pancreatic cancer.”
To conduct his research, Damaghi plans to look at cells in combination by using digital pathology, which can help reveal tumor ecosystems and niches.
He also appreciated the work of Joel Saltz, the Founding Chair in the Department of Biomedical Informatics. “In the fight against cancer, we all need to unite against this nasty disease,” Damaghi said. “From looking at it at different angles, we can understand it first and then design a plan to defeat it.”
Originally from Tehran, Iran, Damaghi is the oldest of five brothers. He said his parents encouraged them to explore their curiosity.
Damaghi, whose wife Narges and two daughters Elissa and Emilia are still in Tampa and hope to join him before long, has hit the ground running at Stony Brook, where he has hired three postdoctoral researchers, a lab manager, four PhD students, two master’s candidates, and three undergraduates.
Damaghi is inspired to conduct cancer research in part because of losses in his family. Two grandparents died from cancer, his aunt has breast cancer, and his cousin, who had cancer when he was 16, fought through the disease and is a survivor for 20 years.
Damaghi bicycles and plays sports including soccer. He also enjoys cooking and said his guests appreciate his Persian kebobs.
As for his arrival in Stony Brook, he said it was “the best option for me. It’s a great package and has everything I need.”