Reducing carbohydrates may be more important than restricting calories
By David Dunaief, M.D.

Triglycerides are part of the lipid, or cholesterol, profile. They get less attention than the other substances, HDL (“good”) and LDL (“bad”) cholesterol, but they’re no less significant.
For 30 years, we have debated whether a high triglyceride level is a biomarker for cardiovascular disease — heart disease and stroke — or an independent risk in its own right (1, 2). Either way, triglycerides are important.
What are they? The most rudimentary explanation is that they are a kind of fat in the blood. They are composed of sugar alcohol and three fatty acids. Thus, it’s no surprise that alcohol, sugars and excess calorie consumption may be converted into triglycerides.
Risk factors for high triglycerides include obesity, smoking, a high carbohydrate diet, uncontrolled diabetes, hypothyroidism (underactive thyroid), cirrhosis (liver disease), excessive alcohol consumption and some medications (3).
What levels are normal? Optimal levels are <100 mg/dL; however, less than 150 mg/dL is considered within normal range. Borderline triglycerides are 150–199 mg/dL, high levels are 200–499 mg/dL, and very high are >500 mg/dL (3).
While medicines that focus on triglycerides, fibrates and niacin can lower them significantly, this reduction may not result in clinical benefits, such as reducing the risk of cardiovascular events. The ACCORD Study, a randomized controlled trial, questioned the effectiveness of medication; when these therapies were added to statins in type 2 diabetes patients, they did not further reduce the risk of cardiovascular disease and events (4). Instead, it seems that lifestyle modifications may be the best way to control triglyceride levels. Let’s look at the evidence.
Exercise — timing and intensity
If you need a reason to exercise, here is a really good one. Study results showed that walking a modest distance with alacrity and light weight training approximately an hour after eating (postprandial) reduced triglyceride levels by 72 percent (5). However, if patients did the same workout prior to eating, postprandial triglycerides were reduced by 25 percent. This is still good, but not as impressive.
Participants walked a modest distance of just over 1 mile (2 kilometers). This was a small pilot study of 10 young healthy adults for a very short duration. The results are intriguing, nonetheless, since there are few data that give specifics on the optimal amount and timing of exercise.
Exercise trumps calorie restriction
There is good news for those who want to lower triglycerides: Calorie restriction may not be the best answer. Instead, we probably should be looking at exercise and carbohydrate intake.
In a well-controlled trial, results showed that those who walked and maintained 60 percent of their maximum heart rate, which is a modest level, showed an almost one-third reduction in triglycerides compared to the control group (maintain caloric intake and no exercise expenditure) (6). Those who restricted their calorie intake saw no difference compared to the control. This was a small study of 11 young adult women. Thus, calorie restriction was trumped by exercise.
Carbohydrate reduction, not calorie restriction
In addition, when calorie restriction was compared to carbohydrate reduction, results showed that carbohydrate reduction was more effective at lowering triglycerides (7). In this small, but well-designed study, patients with nonalcoholic fatty liver disease were randomized to one of two diets, lower calorie (1200–1500 kcal/day) or lower carbohydrate (20 g/day). Both groups lost similar amounts of weight and significantly reduced triglycerides, but the lower carbohydrate group reduced triglycerides by 55 percent versus 28 percent for the lower calorie group. The reason for this difference may have to do with oxidation in the liver and the body as a whole. However, the weakness of this study was its duration of only two weeks.
Fasting versus nonfasting blood tests
The paradigm has been that, when cholesterol levels are drawn, fasting levels provide a more accurate reading. Except this may not be true.
NHANES III data suggest that nonfasting and fasting levels yield similar results related to all-cause mortality and cardiovascular mortality risk. LDL levels were similarly predictive, regardless of whether a patient had fasted or not. The researchers used 4,299 pairs of fasting and nonfasting cholesterol levels. The duration of follow-up was strong, with a mean of 14 years (8).
With regards to stroke risk assessment, nonfasting triglycerides may be more valuable than fasting. In a study involving 13,596 participants, results showed that as nonfasting triglycerides rose, the risk of stroke also rose significantly (9). Compared to those who had levels below 89 mg/dL (the control), those with 89–176 mg/dL had a 1.3-fold increased risk of cardiovascular events, whereas those within the range of 177–265 mg/dL had a twofold increase, and women in the highest group (>443 mg/dL) had an almost fourfold increase. The results were similar for men, with a threefold increase.
The benefit of nonfasting is that it is more realistic and, according to the authors, also involves remnants of VLDL and chylomicrons, other components of the cholesterol profile that interact with triglycerides and may affect the inner part (endothelium) of the arteries.
What have we learned? Triglycerides need to be discussed. Elevated triglycerides may result in heart disease or stroke. The higher the levels, the more likely there will be increased risk of mortality — both all-cause and cardiovascular. Therefore, we ideally should reduce levels to less than 100 mg/dL.
Lifestyle modifications using carbohydrate restriction and modest levels of exercise after a meal may achieve the best results, though the studies are small and need more research. Nonfasting levels may be as important as fasting levels when it comes to triglycerides and the cholesterol profile as a whole; they potentially give a more realistic view of cardiovascular risk, since we don’t live in a vacuum and fast all day.
References:
(1) Circulation. 2011;123:2292-2333. (2) N Engl J Med. 1980;302:1383–1389. (3) nlm.nih.gov. (4) N Engl J Med. 2010;362:1563-1574. (5) Med Sci Sports Exerc. 2013;45(2):245-252. (6) Med Sci Sports Exerc. 2013;45(3):455-461. (7) Am J Clin Nutr. 2011;93(5):1048-1052. (8) Circulation Online. 2014 July 11. (9) JAMA 2008;300:2142-2152.
Dr. Dunaief is a speaker, author and local lifestyle medicine physician focusing on the integration of medicine, nutrition, fitness and stress management.
٭We invite you to check out our new weekly Medical Compass MD Health Videos on Times Beacon Record News Media’s website, www.tbrnewsmedia.com










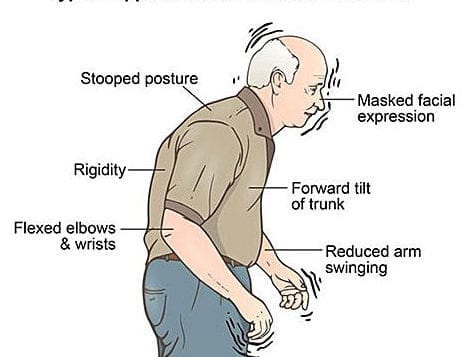
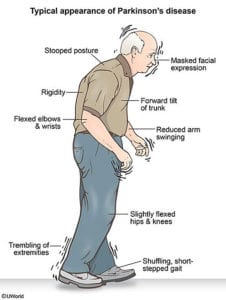 When we typically think of using CoQ10, a coenzyme found in over-the-counter supplements, it is to compensate for depletion from statin drugs or due to heart failure. Doses range from 100 to 300 mg. However, there is evidence that CoQ10 may be beneficial in Parkinson’s at much higher doses.
When we typically think of using CoQ10, a coenzyme found in over-the-counter supplements, it is to compensate for depletion from statin drugs or due to heart failure. Doses range from 100 to 300 mg. However, there is evidence that CoQ10 may be beneficial in Parkinson’s at much higher doses.



