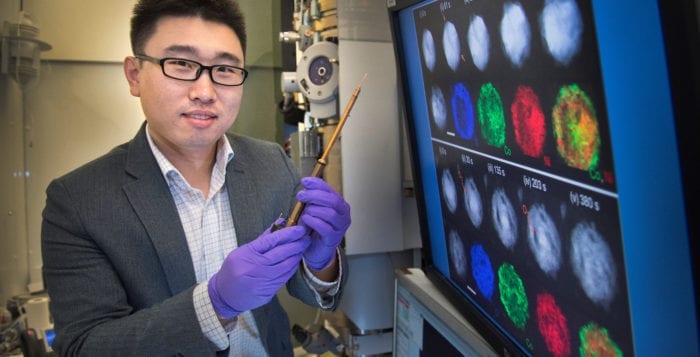
‘Off the charts talented,’ BNL’s Xin makes catalyst discovery
By Daniel Dunaief
The unexpected appearance of Swiss cheese may be preferable to the predicted presence of a balloon. When it comes to the creation of catalysts for fuel-cell-powered vehicles, the formation of a structure that has miniature holes in it may reduce costs and improve energy efficiency.
Using a state-of-the-art facility where he also supports the work of other scientists around the world, Huolin Xin, an associate materials scientist at the Center for Functional Nanomaterials at Brookhaven National Laboratory, recently made the discovery about the structure of a cheaper catalyst. Xin and his collaborators published their work in Nature Communications.
The finding “goes against conventional wisdom,” Xin said. “If you have a precursor that’s nanometers in size that’s a metal and you heat it up in oxygen, normally, it would grow into a hollow structure, like a balloon.” Instead, Xin and his colleagues discovered that mixing nickel and cobalt produces a structure that has porosity but is more like spherical Swiss cheese than a balloon. The new architecture has more material crammed into a smaller region than the hollow balloon. It is also stronger, creating a broader range of potential applications.
Scientists at Brookhaven and at other institutions around the world are seeking ways to take advantage of the growing field of nanotechnology, in which physical, electrical or other types of interactions differ from the macromolecular world of hammers, nails and airplane wings. These nanomaterials take advantage of the high surface area to volume ratio, which offers promise for future technologies. What that means is that these materials contain numerous surfaces without taking up much space, like an intricate piece of origami, or, in Xin’s case, a sphere with higher packing density.
The potential new catalyst could be used as a part of an oxygen reduction reaction in an alkaline environment. In a car that uses hydrogen, the reaction would produce water with zero emissions, Xin said. To see the structure of this catalyst, Xin used environmental transmission electron microscopy and electron tomography. The TEM uses computed axial tomography. This is similar to the CAT scan in a hospital, except that the sample Xin studied was much smaller, about 100 nanometers in size, which is 100 times thinner than the width of a human hair.
In addition to determining and defining the structure of the final product, scientists are trying to understand the process that led to that configuration. They can use the environmental transmission electron microscope, which allows gas flowing to study the formation of the catalyst.
Charles Black, the director at the Center for Functional Nanomaterials, said Xin is “off the charts talented” and is a “world leader” in figuring out ways to get more information from the electron microscope. Xin, Black said, has helped create a three-dimensional picture by tilting a two-dimensional sample at different angles in the microscope. “He had already made great strides in improving the speed with which this could be done,” Black said. “He’s also improved the process to the point where you don’t have to be a super expert to do it anymore.”
By slowing the reaction in the nickel-cobalt catalyst down and studying how it forms, Xin uncovered that the shell is not solid: It has pinholes. Once those small holes form, the oxygen infiltrates the pores. The process repeats itself, as shells form, then break up, then oxygen forms another shell, which breaks up, until the process leads to a spherically stacked collection of Swiss cheese structures. The process is ready for industrial-scale applications, Xin said, because the whole synthesis involves putting the elements into a furnace and baking it. While this could have applications in fuel cells, the catalyst still awaits a breakthrough technology with alkaline fuel cells.
The technological breakthrough Xin awaits is an alkaline membrane that can conduct a hydroxyl group. “We are definitely doing research for the future,” he said. “We’re still awaiting the essential element, which is the ionic conductive membrane, to become a technologically mature product.” Xin isn’t focused on creating that membrane, which is a task for organic chemists. Instead, his main focus is on inorganic materials.
As a member of the BNL staff at the Center for Functional Nanomaterials, which is a facility that provides technical support to other scientists, Xin spends half of his time with other researchers on the TEM and half of his time on his own research. “We really have been fortunate to have found someone like [Xin] who wants to excel in both sides of his mission,” Black said “Someone as talented as [Xin], who is very smart with big ideas and increasingly ambitious in terms of what he wants to accomplish for himself … checks his ego at the door and he helps others accomplish their goals.” To improve his ability as a colleague, Xin reads about what the users of the TEM are doing and talks with them about their work.
Xin has been working at BNL for over three years. When he’s not in the lab, Xin enjoys traveling to snorkel in the U.S. Virgin Islands, including his favorite destination, St. John. A skier, Xin’s favorite winter recreational mountain is Lake Placid. Xin grew up in Beijing, where his father is a professor in a business school and his mother is an engineer. He appreciates the opportunity to engage in a broad universe of fields through the work he does at BNL and appreciates the scientific partnerships he’s formed. “My primary focus is on creating novel microscope techniques that can advance the electron microscopy field,” he explained. “I apply them to a variety of materials projects.” Xin estimates that half of his materials application projects come from collaborators.