By David Dunaief, M.D.

Parkinson’s disease (PD) is the second most common neurodegenerative disorder in the U.S. after Alzheimer’s disease. Estimates put the number of people living with Parkinson’s disease at up to 1.2 million, with 90,000 new diagnoses each year (1).
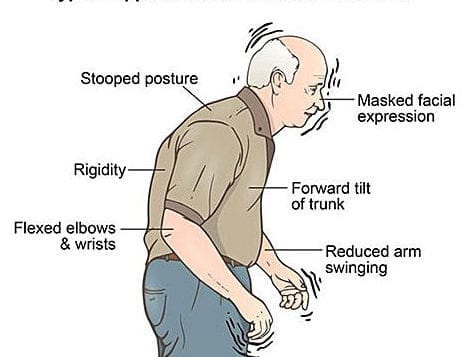
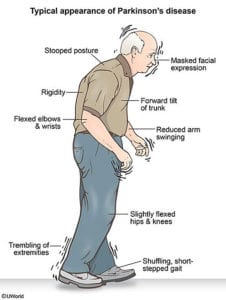
Patients with PD suffer from a collection of symptoms caused by the breakdown of brain neurons. There’s a lot we still don’t know about the causes of PD; however, risk factors may include head trauma, genetics, exposure to toxins and heavy metals, and lifestyle issues, like lack of exercise.
The part of the brain most affected is the basal ganglia, and the prime culprit is dopamine deficiency that occurs in this brain region (2). Adding back dopamine has been the mainstay of medical treatment, but eventually the neurons themselves break down, and the medication becomes less effective.
Is there hope? Yes, in the form of medications and deep brain stimulatory surgery, but also by modifying lifestyle, considering factors like iron, vitamin D, inflammation, and CoQ10. While the research is not conclusive, it is intriguing and gives us more options.
What impact does iron have on the brain?
This heavy metal is potentially harmful for neurodegenerative diseases such as Alzheimer’s disease, macular degeneration, multiple sclerosis and, yes, Parkinson’s disease. The problem is that it can cause oxidative damage.
In a small, yet well-designed, randomized controlled trial (RCT), researchers used a chelator to remove iron from the substantia nigra, a specific part of the brain where iron breakdown may be dysfunctional. An iron chelator is a drug that removes the iron. Here, deferiprone (DFP) was used at a modest dose of 30 mg/kg/d (3).
The chelator reduced the risk of disease progression significantly on the Unified Parkinson Disease Rating Scale (UPDRS) during the 12-month study. Participants who were treated sooner had lower levels of iron compared to a group that used the chelator six months later. A specialized MRI was used to measure the brain’s iron levels.
The iron chelator does not affect, nor should it affect, systemic levels of iron, only those in the substantia nigra region of the brain. The chelator may work by preventing degradation of the dopamine-containing neurons. Your physician may also recommend that you consume foods that contain less iron.
What is the role of inflammation in PD?
In a recent study, researchers tested 58 newly diagnosed PD participants’ blood and compared their results to 62 healthy control participants (4). Some of the PD arm participants had additional testing done, including cerebrospinal fluid samples and brain imaging. All these tests were looking for specific inflammatory markers.
Researchers found that those with PD had significantly higher brain inflammation levels than those without PD in specific regions. Their blood and cerebrospinal fluid also had high inflammatory markers. These measures correlated with worse visuospatial and cognitive scores.
While this study provides hints of possible treatments, we need additional studies to identify whether the inflammation is a cause or an effect of PD.
Regardless, adopting a low-inflammatory foods diet might help mitigate some symptoms of PD or slow its advancement.
Does CoQ10 help slow PD progression?
There is evidence that CoQ10 may be beneficial in PD at high doses.
In an RCT, results showed that those given 1,200 mg of CoQ10 daily reduced the progression of the disease significantly based on UPDRS changes, compared to a placebo group (5). Other doses of 300 and 600 mg showed trends toward benefit, but were not significant. This was a 16-month trial in a small population of 80 patients. Unfortunately, results for other CoQ10 studies have been mixed.
In this study, CoQ10 was well-tolerated at even the highest dose. Thus, there may be no downside to trying CoQ10 in those with PD.
Does Vitamin D make a difference?
Vitamin D may play dual roles of both reducing the risk of Parkinson’s disease and slowing its progression.
In a prospective study of over 3000 patients, results show that vitamin D levels measured in the highest quartile reduced the risk of developing Parkinson’s disease by 65 percent, compared to the lowest quartile (6). This is impressive, especially since the highest quartile patients had vitamin D levels that were what we qualify as insufficient, with blood levels of 20 ng/ml, while those in the lowest quartile had deficient blood levels of 10 ng/ml or less.
In an RCT with 121 patients, results showed that 1,200 IU of vitamin D taken daily may have reduced the progression of PD significantly on the UPDRS compared to a placebo over a 12-month duration (7). Also, this amount of vitamin D increased the blood levels by almost two times from 22.5 to 41.7 ng/ml.
In a 2019 study of 182 PD patients and 185 healthy control subjects, researchers found that higher serum vitamin D levels correlated to reduced falls and alleviation of other non-motor PD symptoms (8).
Vitamin D research is ongoing, as this all seems promising.
So, what are our takeaways? Though medication is the gold standard for Parkinson’s disease treatment, lifestyle modifications can have a significant impact on both its prevention and treatment. Each lifestyle change in isolation may have modest effects, but cumulatively their impact could be significant.
References:
(1) parkinson.org. (2) uptodate.com. (3) Antioxid Redox Signal. 2014;10;21(2):195-210. (4) Movement Disorders. 2023;38;5:743-754. (5) Arch Neurol. 2002;59(10):1541-1550. (6) Arch Neurol. 2010;67(7):808-811. (7) Am J Clin Nutr. 2013;97(5):1004-1013. (8) Neurologica. 2019;140(4):274-280.
Dr. David Dunaief is a speaker, author and local lifestyle medicine physician focusing on the integration of medicine, nutrition, fitness and stress management. For further information, visit www.medicalcompassmd.com or consult your personal physician.










 When we typically think of using CoQ10, a coenzyme found in over-the-counter supplements, it is to compensate for depletion from statin drugs or due to heart failure. Doses range from 100 to 300 mg. However, there is evidence that CoQ10 may be beneficial in Parkinson’s at much higher doses.
When we typically think of using CoQ10, a coenzyme found in over-the-counter supplements, it is to compensate for depletion from statin drugs or due to heart failure. Doses range from 100 to 300 mg. However, there is evidence that CoQ10 may be beneficial in Parkinson’s at much higher doses.


